Acid Base Balance Made Easy for Nurses
Interpretation of arterial blood gases (ABGs) is a crucial skill that a lot of student nurses and medical practitioners need to learn. In this guide, we'll help you understand the concepts behind arterial blood gas and teach you the easiest and most fun way to interpret ABGs using the tic-tac-toe method.
What is arterial blood gas?

An arterial blood gas is a laboratory test to monitor the patient's acid-base balance. It is used to determine the extent of the compensation by the buffer system and includes the measurements of the acidity (pH), levels of oxygen, and carbon dioxide in arterial blood. Unlike other blood samples obtained through a vein, a blood sample from an arterial blood gas (ABG) is taken from an artery (commonly on radial or brachial artery).
What are the components of arterial blood gas?
There are six components of arterial blood gas (ABGs):
pH
The pH is the concentration of hydrogen ions and determines the acidity or alkalinity of body fluids. A pH of 7.35 indicates acidosis and a pH greater than 7.45 indicates alkalosis.The normal ABG level for pH is 7.35 to 7.45.
PaCO2 (Partial Pressure of Carbon Dioxide)
PaCO2 or partial pressure of carbon dioxide shows the adequacy of the gas exchange between the alveoli and the external environment (alveolar ventilation). Carbon dioxide (CO2) cannot escape when there is damage in the alveoli, excess CO2 combines with water to form carbonic acid (H2CO3) causing an acidotic state. When there is hypoventilation in the alveolar level (for example, in COPD), the PaCO2 is elevated, and respiratory acidosis results. On the other hand, when there is alveolar hyperventilation (e.g., hyperventilation), the PaCO2 is decreased causing respiratory alkalosis. For PaCO2, the normal range is 35 to 45 mmHg (respiratory determinant).
PaO2 (Partial Pressure of Oxygen)
PaO2 or partial pressure of oxygen or PAO2 indicates the amount of oxygen available to bind with hemoglobin. The pH plays a role in the combining power of oxygen with hemoglobin: a low pH means there is less oxygen in the hemoglobin. For PaO2, the normal range is 75 to 100 mmHg
SO2 (Oxygen Saturation)
SO2 or oxygen saturation, measured in percentage, is the amount of oxygen in the blood that combines with hemoglobin. It can be measured indirectly by calculating the PAO2 and pH Or measured directly by co-oximetry. Oxygen saturation, the normal range is 94–100%
HCO3 (Bicarbonate)
HCO3 or bicarbonate ion is an alkaline substance that comprises over half of the total buffer base in the blood. A deficit of bicarbonate and other bases indicates metabolic acidosis. Alternatively, when there is an increase in bicarbonates present, then metabolic alkalosis results.
BE (Base Excess)
BE. Base excess or BE value is routinely checked with HCO3 value. A base excess of less than –2 is acidosis and greater than +2 is alkalosis. Base excess, the normal range is –2 to +2 mmol/L
Normal Values in Arterial Blood Gas
To determine acid-base imbalance, you need to know and memorize these values to recognize what deviates from normal. The normal range for ABGs is used as a guide, and the determination of disorders is often based on blood pH. If the blood is basic, the HCO3 level is considered because the kidneys regulate bicarbonate ion levels. If the blood is acidic, the PaCO2 or partial pressure of carbon dioxide in arterial blood is assessed because the lungs regulate the majority of acid. The normal ABG values are the following:
- For pH, the normal range is 7.35 to 7.45
- For PaCO2, the normal range is 35 to 45 mmHg (respiratory determinant)
- For PaO2, the normal range is 75 to 100 mmHg
- For HCO3, the normal range is 22 to 26 mEq/L (metabolic determinant)
- Oxygen saturation, the normal range is 94–100%
- Base excess, the normal range is –2 to +2 mmol/L
Interpreting Arterial Blood Gas Imbalances
Interpreting arterial blood gases is used to detect respiratory acidosis or alkalosis, or metabolic acidosis or alkalosis during an acute illness. To determine the type of arterial blood gas the key components are checked. The best (and fun) way of interpreting arterial blood gas is by using the tic-tac-toe method below:
Goals of Arterial Blood Gas analysis

For the purpose of this guide, we have set three (3) goals that we need to accomplish when interpreting arterial blood gases. The goals are as follows:
- Based on the given ABG values, determine if values interpret ACIDOSIS or ALKALOSIS.
- Second, we need to determine if values define METABOLIC or RESPIRATORY.
- Lastly, we need to determine the compensation if it is: FULLY COMPENSATED, PARTIALLY COMPENSATED, or UNCOMPENSATED.
We need to keep these goals in mind as they'll come up later in the steps for the ABG interpretation technique.
Steps in ABG analysis using the tic-tac-toe method
There are eight (8) steps simple steps you need to know if you want to interpret arterial blood gases (ABGs) results using the tic-tac-toe technique.
1. Memorize the normal values.
The first step is you need to familiarize yourself with the normal and abnormal ABG values when you review the lab results. They are easy to remember:
- For pH, the normal range is 7.35 to 7.45
- For PaCO2, the normal range is 35 to 45
- For HCO3, the normal range is 22 to 26
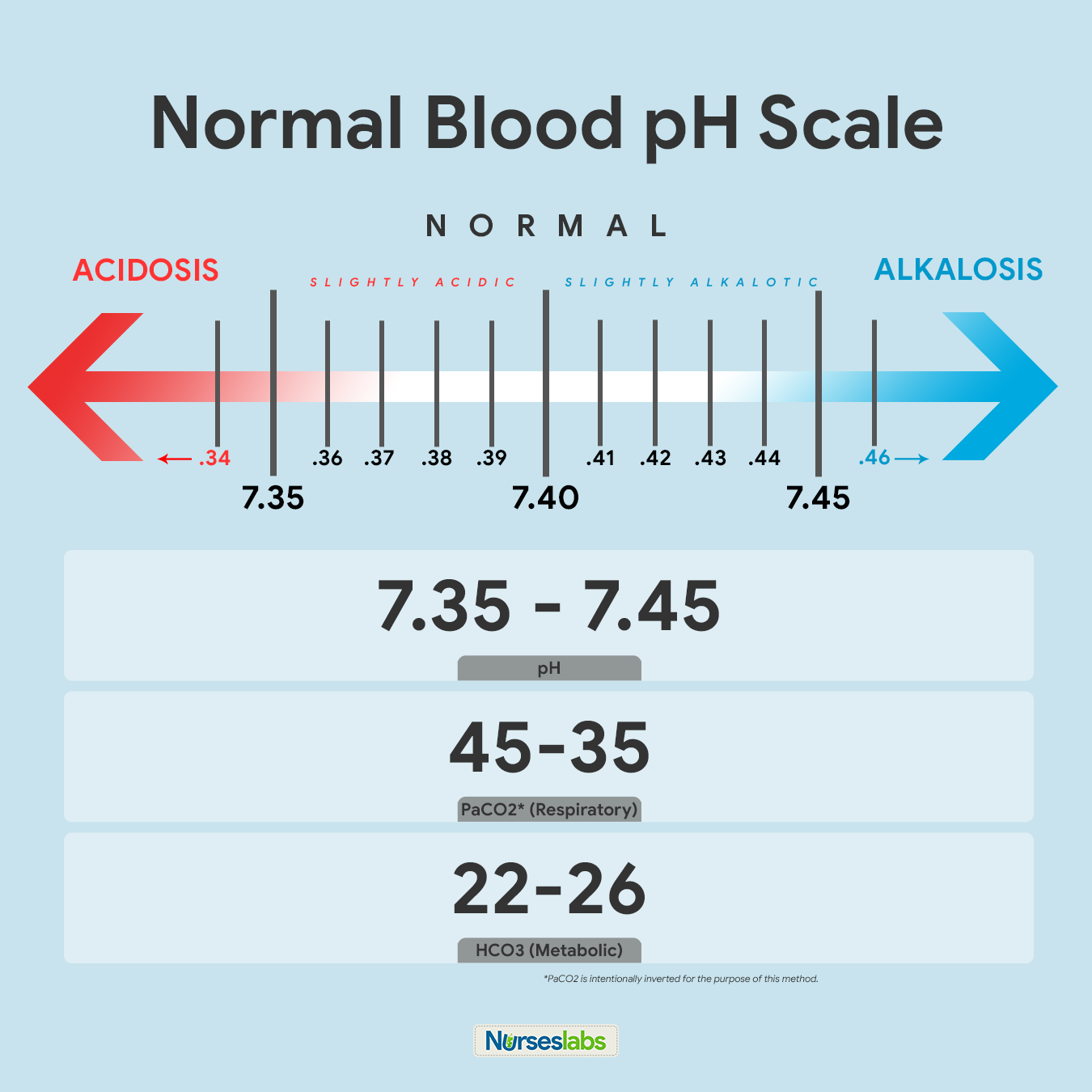
The recommended way of memorizing it is by drawing the diagram of normal values above. Write it down together with the arrows indicating ACIDOSIS or ALKALOSIS. Note that PaCO2 is intentionally inverted for the purpose of the Tic-Tac-Toe method.
2. Create your tic-tac-toe grid.
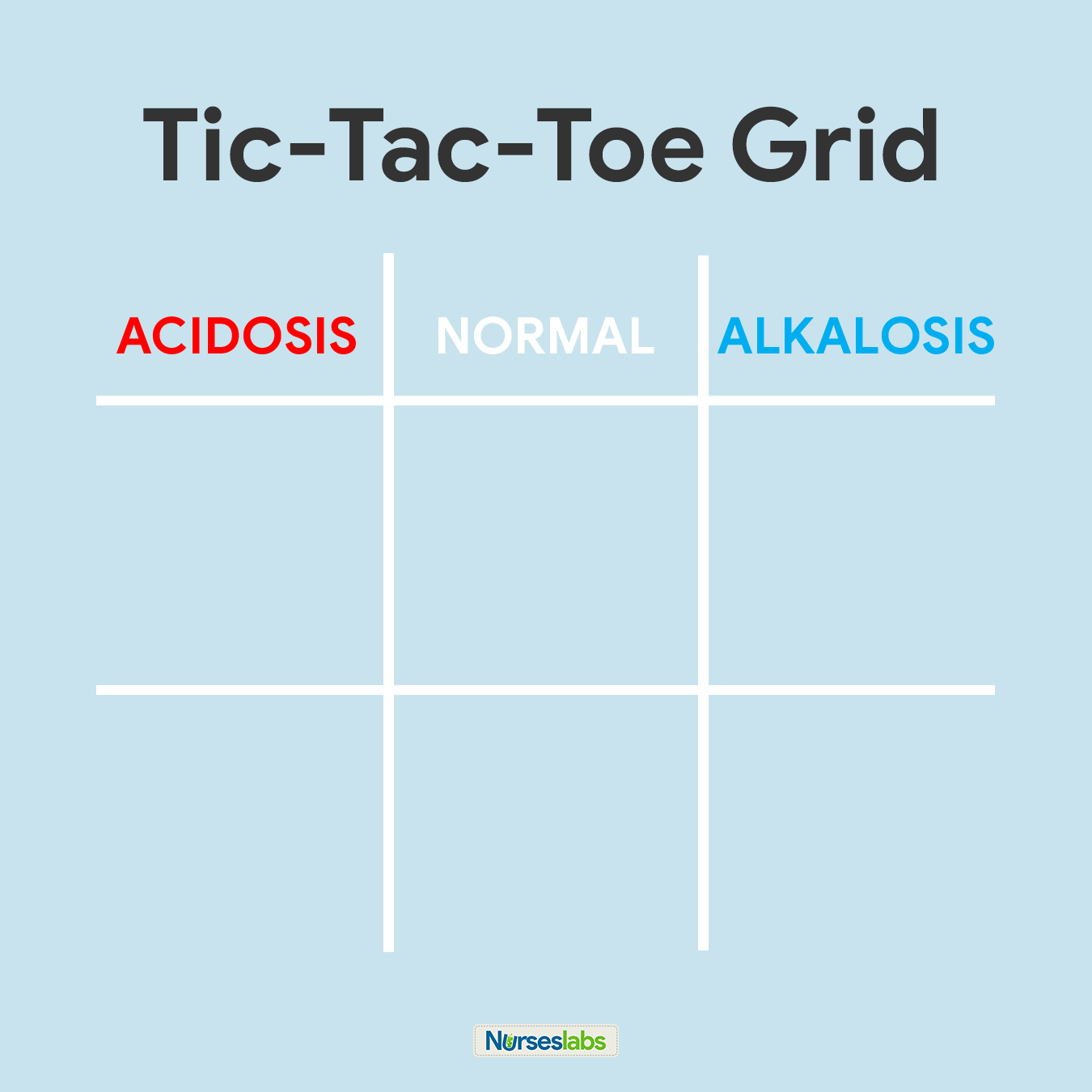
Once you've memorized the normal values and the diagram, create a blank your tic-tac-toe grid and label the top row as ACIDOSIS, NORMAL, and ALKALOSIS. Based on their values, we need to determine in which column we'll place pH, PaCO2, and HCO3 in the grid.
3. Determine if pH is under NORMAL, ACIDOSIS, or ALKALOSIS.
The third step of this technique is to determine the acidity or alkalinity of the blood with the given value of the pH as our determining factor. Remember in step #1 that the normal pH range is from 7.35 to 7.45.
- If the blood pH is between 7.35 to 7.39, the interpretation is NORMAL but SLIGHTLY ACIDOSIS, place it under the NORMAL column.
- If the blood pH is between 7.41 to 7.45, interpretation is NORMAL but SLIGHTLY ALKALOSIS, place it under the NORMAL column.
- Any blood pH below 7.35 (7.34, 7.33, 7.32, and so on…) is ACIDOSIS, place it under the ACIDOSIS column.
- Any blood pH above 7.45 (7.46, 7.47, 7.48, and so on…) is ALKALOSIS, place it under the ALKALOSIS column.
Please use the diagram below to help you visualize whether the normal value is ACIDOSIS or ALKALOSIS.
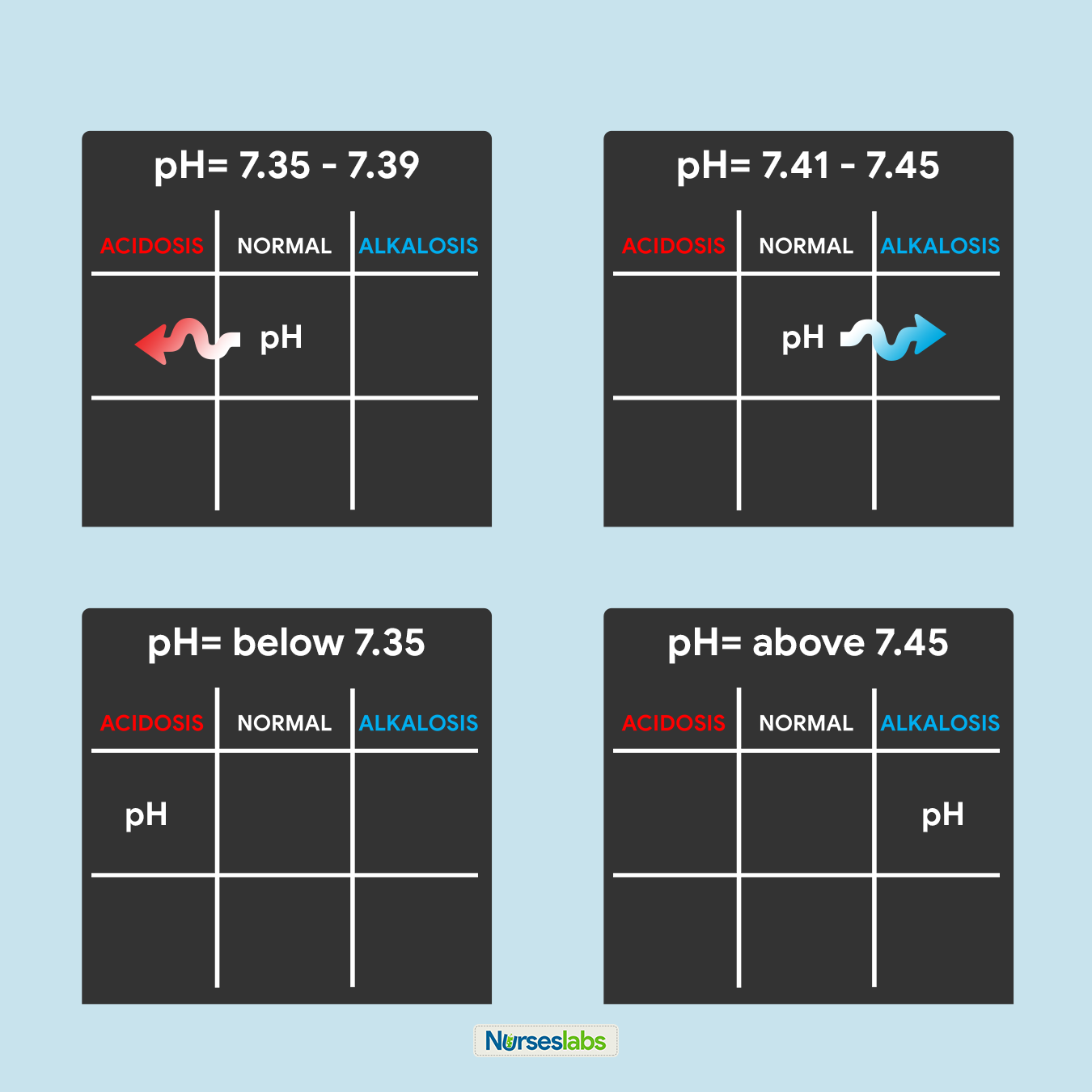
Once you've determined whether the pH is under the ACIDOSIS or ALKALOSIS, plot it on your tic-tac-toe grid under the appropriate column.
4. Determine if PaCO2 is under NORMAL, ACIDOSIS, or ALKALOSIS.
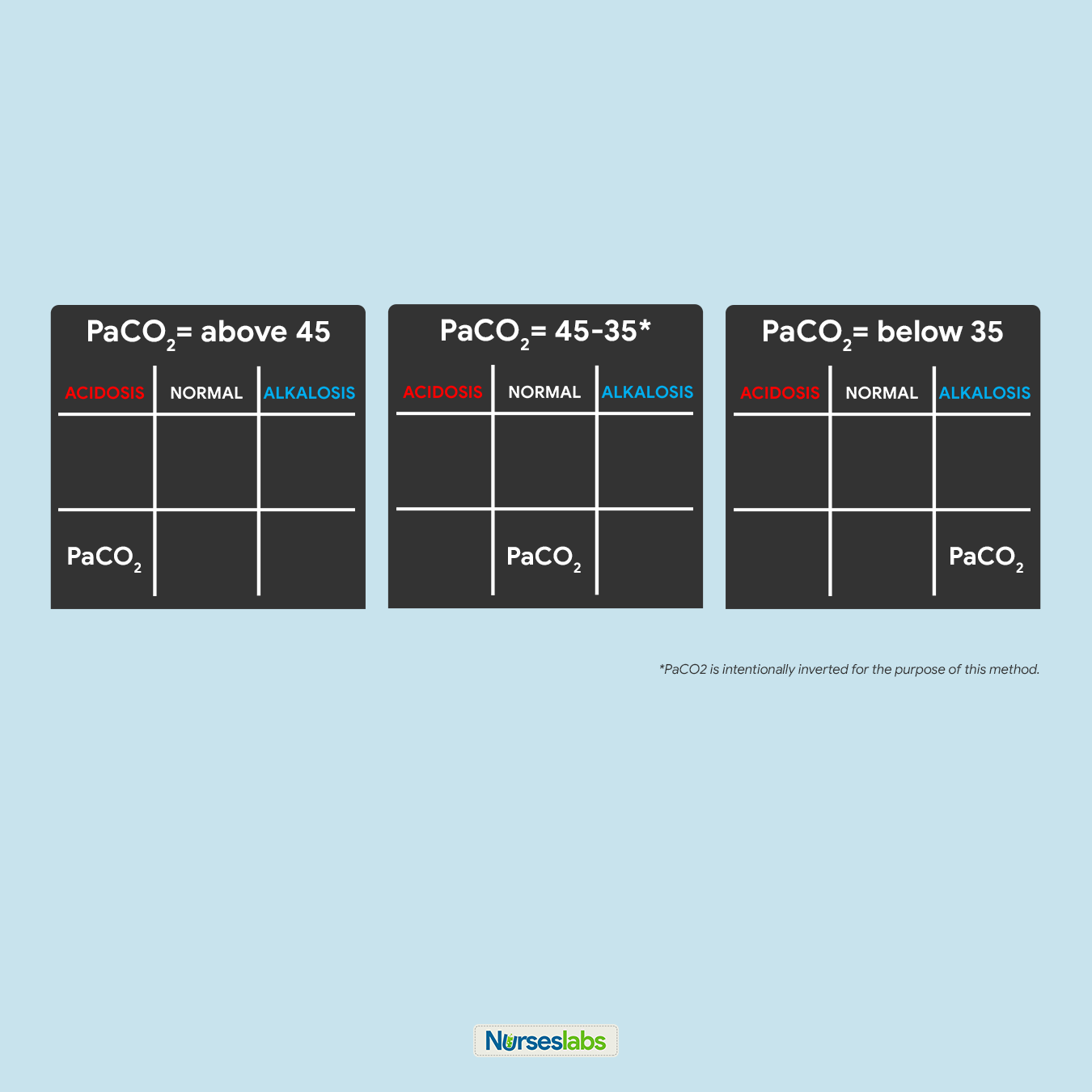
For this step, we need to interpret if the value of PaCO2 is within the NORMAL range, ACIDIC, or BASIC and plot it on the grid under the appropriate column. Remember that the normal range for PaCO2 is from 35 to 45:
- If PaCO2 is below 35, place it under the ALKALOSIS column.
- If PaCO2 is above 45, place it under the ACIDOSIS column.
- If PaCO2 is within its normal range, place it under the NORMAL column.
5. Determine if HCO3 is under NORMAL, ACIDOSIS, or ALKALOSIS.

Next, we need to interpret if the value of HCO3 is within the NORMAL range, ACIDIC, or BASIC and plot it under the appropriate column in the tic-tac-toe grid. Remember that the normal range for HCO3 is from 22 to 26:
- If HCO3 is below 22, place it under the ACIDOSIS column.
- If HCO3 is above 26, place it under the ALKALOSIS column.
- If HCO3 is within its normal range, place it under the NORMAL column.
6. Solve for goal #1: ACIDOSIS or ALKALOSIS.
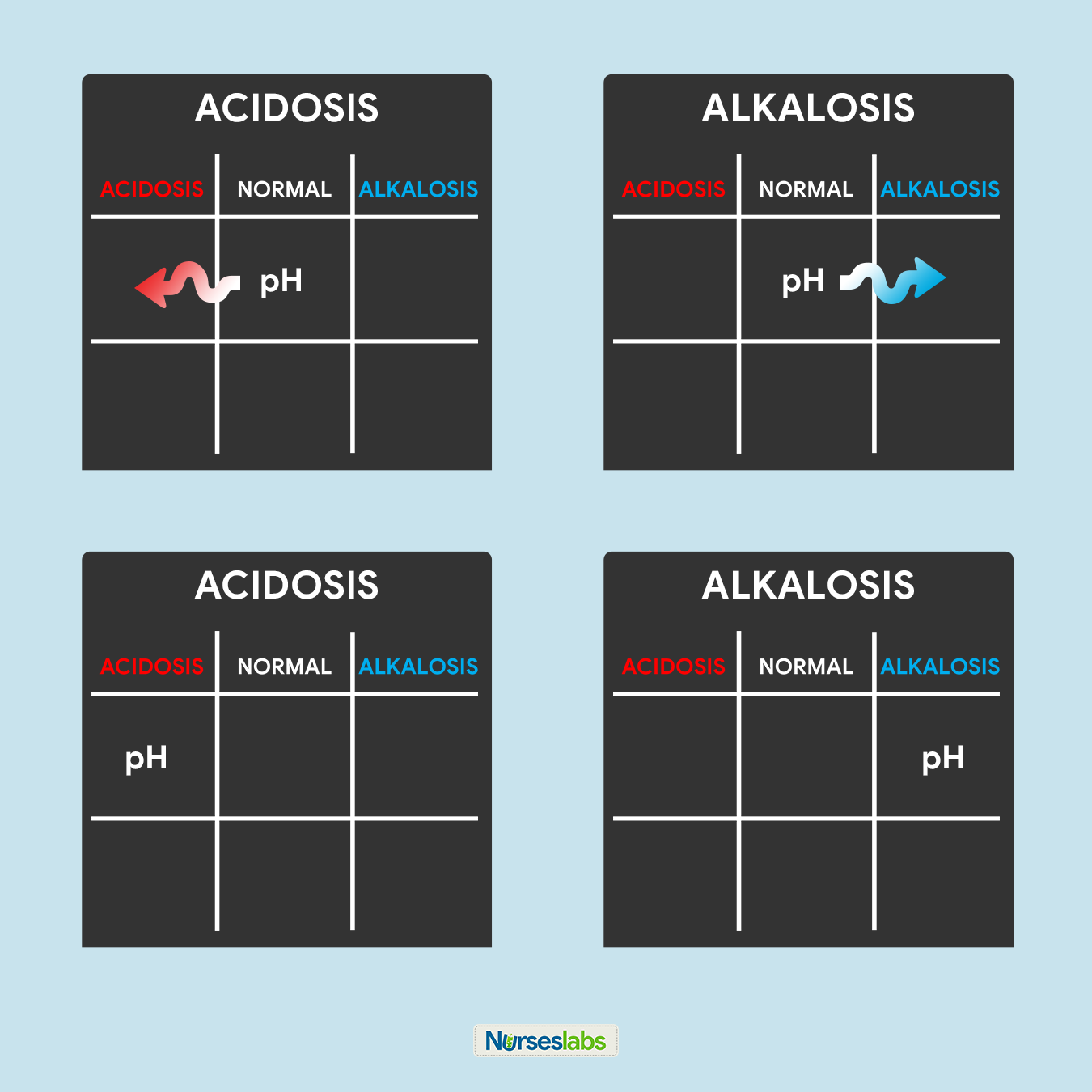
Now, we will start solving for our goals. Looking at the tic-tac-toe grid, determine whether in what column the pH is placed and interpret the results:
- If pH is under the ACIDOSIS column, it is ACIDOSIS.
- If pH is under the ALKALOSIS column, it is ALKALOSIS.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret accordingly.
In this step, we can accomplish goal #1 of determining ACIDOSIS or ALKALOSIS.
7. Solve for goal #2: METABOLIC or RESPIRATORY.

Looking back again on the tic-tac-toe grid, determine if pH is under the same column as PaCO2 or HCO3 so we can accomplish our goal #2 of determining if the ABG is RESPIRATORY or METABOLIC. Interpret the results as follows:
- If pH is under the same column as PaCO2, it is RESPIRATORY.
- If pH is under the same column as HCO3, it is METABOLIC.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret accordingly.
8. Solve for goal #3: COMPENSATION.
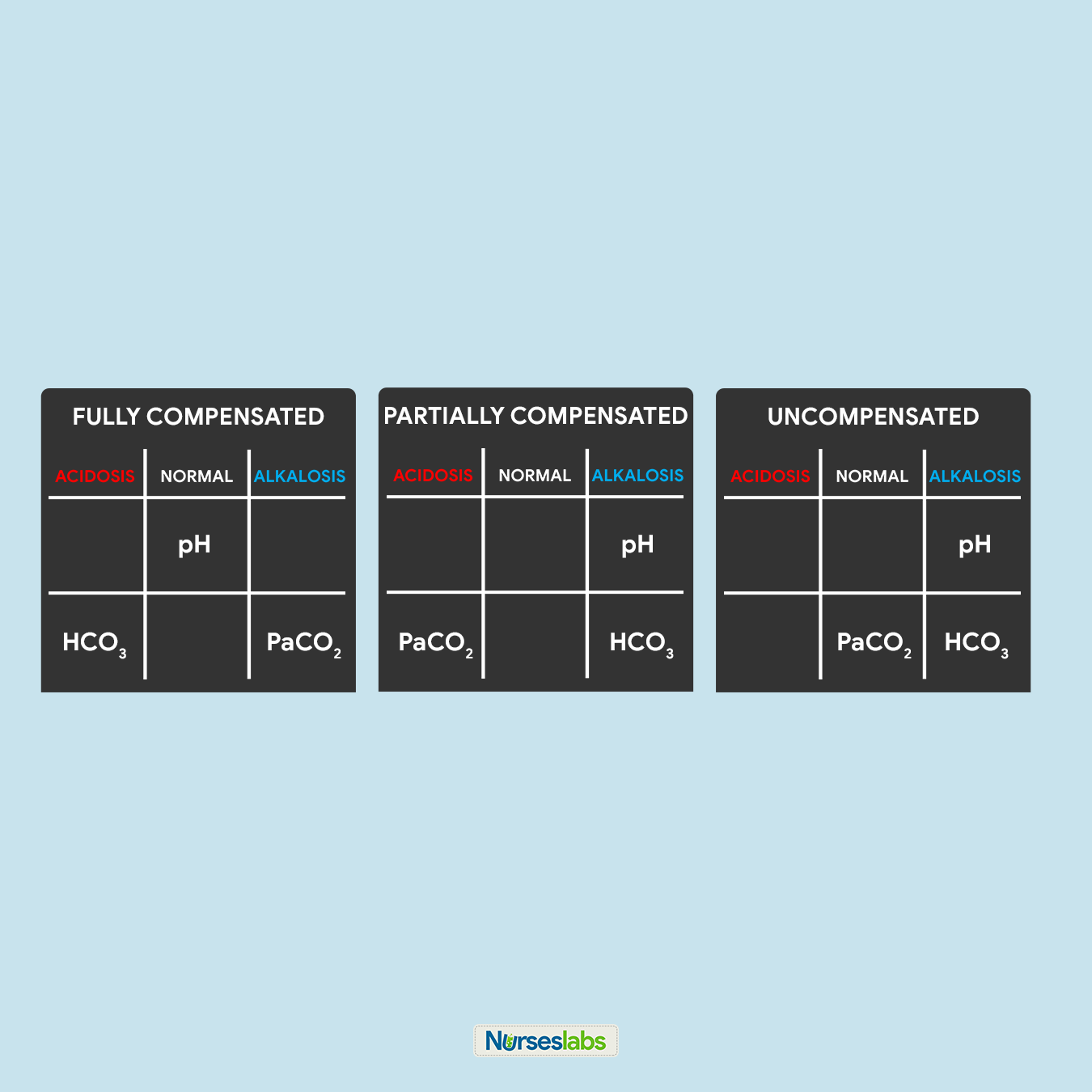
Lastly, we need to determine the compensation to accomplish our goal #3. Interpret the results as follows:
- It is FULLY COMPENSATED if pH is normal.
- It is PARTIALLY COMPENSATED if all three (3) values are abnormal.
- It is UNCOMPENSATED if PaCO2 or HCO3 is normal and the other is abnormal.
Application and Examples
Let's solve for the ABG interpretation with the examples below:
Practice Problem #1:
pH=7.26 | PaCO2=32 | HCO3=18
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of 7.26 ABNORMAL and under ACIDOSIS, so we place pH under ACIDOSIS.
- PaCO2 of 32 is ABNORMAL and under ALKALOSIS, so we place PaCO2 under ALKALOSIS.
- HCO3 of 18 is ABNORMAL and under ACIDOSIS, so we place HCO3 under ACIDOSIS.
- pH is under ACIDOSIS, therefore solving for goal #1, we have ACIDOSIS.
- pH is on the same column as HCO3, therefore solving for goal #2, we have METABOLIC.
- All three values are ABNORMAL, therefore solving for goal #3, we have a PARTIALLY COMPENSATED ABG.
The answer to Practice Problem #1:
Metabolic Acidosis, Partially Compensated
Practice Problem #2:
pH=7.44 | PaCO2=30 | HCO3=21
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of 7.44 is NORMAL but slightly leaning towards ALKALOSIS, so we place pH under the NORMAL column with an arrow pointing towards the ALKALOSIS column.
- PaCO2 of 30 is ABNORMAL and ALKALOSIS, so we place PaCO2 under the ALKALOSIS column.
- HCO3 of 21 is ABNORMAL and ACIDOSIS, so we place HCO3 under the ACIDOSIS column.
- pH of 7.44 is NORMAL but leaning towards ALKALOSIS, therefore solving for goal #1, we have ALKALOSIS.
- pH is NORMAL but is leaning towards ALKALOSIS, therefore under the same column as PaCO2. Solving for goal #2, we have RESPIRATORY.
- pH is NORMAL, therefore solving for goal #3, we have a FULLY COMPENSATED ABG.
The answer to Practice Problem #2:
Respiratory Alkalosis, Fully Compensated
Practice Problem #3:
pH=7.1 | PaCO2=40 | HCO3=18
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of 7.1 is ABNORMAL and ACIDOSIS, therefore, we place pH under the ACIDOSIS column in the tic-tac-toe grid.
- PaCO2 of 40 is NORMAL, therefore, place it under the NORMAL column.
- HCO3 of 18 is ABNORMAL and ACIDOSIS, so we place HCO3 under the ACIDOSIS column.
- pH of 7.1 is ACIDOSIS, therefore, solving for goal #1, we have ACIDOSIS.
- pH is under the same column as HCO3, therefore, solving for goal #2, we have determined that it is METABOLIC.
- pH is ABNORMAL so as HCO3, but PaCO3 is under the NORMAL column. Solving for goal #3, we can interpret it as UNCOMPENSATED.
The answer to Practice Problem #3:
Metabolic Acidosis, Uncompensated
How to draw Arterial Blood Gas?
Arterial blood is usually drawn via the brachial or radial artery.
- Inform that client about the procedure and that there is no food or fluid restriction imposed.
- Note if the client is taking anticoagulant therapy or aspirin as this may affect results.
- Note if the client is receiving oxygen therapy (flow rate, type of administration device), and the client's current temperature.
- Using a heparinized needle and syringe, collect 1 to 5 mL of arterial blood. Common sites for drawing arterial blood are the radial and brachial artery.
- Put the syringe with arterial blood in an ice-water bag to minimize the metabolic activity of the sample.
- Deliver the blood sample immediately to the laboratory.
- Apply pressure to the puncture site for 5 minutes or longer.
Acid-Base Balance and Imbalances
Acid-base imbalances develop when a person's normal homeostatic mechanisms are dysfunctional or overwhelmed. One type of acid-base imbalance is acidosis wherein the blood is relatively too acidic (low pH). The body produces two types of acid, therefore, there are two types of acidosis: respiratory acidosis and metabolic acidosis. On the contrary, alkalosis is a condition wherein the blood is relatively too basic (high pH), there are also two types of alkalosis: respiratory alkalosis and metabolic alkalosis.
When acid-base imbalances occur, the body activates its compensatory mechanisms (the lungs and kidneys) to help normalize the blood pH. The kidneys compensate for respiratory acid-base imbalances while the respiratory system compensates for metabolic acid-base imbalances. This does not correct the root cause of the problem, if the underlying condition is not corrected, these systems will fail.
Respiratory Acidosis
Respiratory acidosis occurs when breathing is inadequate (alveolar hypoventilation) and the lungs are unable to excrete enough CO2 causing PaCO2 or respiratory acid builds up. The extra CO2 combines with water to form carbonic acid, causing a state of acidosis — a common occurrence in emphysema. The kidneys activate its compensatory process (albeit slow, often 24 hours or more) by increasing the excretion of metabolic acids through urination, which increases blood bicarbonate.
Types of Respiratory Acidosis
There are two forms of respiratory acidosis: Acute and Chronic.
- Acute respiratory acidosis. This form of respiratory acidosis occurs immediately. Left untreated, symptoms will get progressively worse. It's a medical emergency and can become life-threatening.
- Chronic respiratory acidosis. This form of respiratory acidosis develops through time. It doesn't cause symptoms. Instead, the body adapts to the increased acidity. For example, the kidneys produce more bicarbonate to help maintain balance. Chronic respiratory acidosis may not cause symptoms. Developing another illness may cause chronic respiratory acidosis to worsen and become acute respiratory acidosis.
Risk Factors
Respiratory acidosis is typically caused by an underlying disease or condition. This is also called respiratory failure or ventilatory failure.
- Hypoventilation. A decrease in ventilation increases the concentration of carbon dioxide in the blood and decreases the blood's pH (brain trauma, coma, hypothyroidism: myxedema).
- Chronic Obstructive Pulmonary Disease (COPD). In chronic respiratory acidosis in COPD patients, the body tries to compensate by retaining more bicarbonate to overcome acidosis.
- Respiratory Conditions. The lungs are not able to eliminate enough of the carbon dioxide produced by the body. Excess carbon dioxide causes the pH of the blood and other bodily fluids to decrease, making them too acidic. (pneumothorax, pneumonia, status asthmaticus)
- Drug Intake. Overdose of an opiate or opioid, such as morphine, tramadol, heroin, fentanyl, or magnesium sulfate (MgSO4) can cause respiratory acidosis.
Signs and Symptoms
Signs and symptoms of respiratory acidosis are as follows:
- Altered level of consciousness. Respiratory acidosis may be the result of an altered level of consciousness caused by encephalopathy or cerebral edema.
- Confusion. Acute respiratory acidosis may also cause symptoms involving the brain, including confusion, stupor, drowsiness, and muscle jerks.
- Disorientation. Respiratory acidosis may result in disorientation, headache, or even focal neurologic signs.
- Coma. When the lungs can't remove all of the carbon dioxide produced by the body through normal metabolism, the blood becomes acidified, leading to increasingly serious symptoms, from sleepiness to coma.
- Tremors. Manifest as shaking or jerking muscle movements.
- Asterixis. An inability to maintain the posture of part of the body.
Management of Respiratory Acidosis
Medical and nursing management of an arterial blood gas of respiratory acidosis includes the following:
- Treat underlying conditions.
- Medications. Bronchodilator medicines and corticosteroids may be used to reverse some types of airway obstruction, like those linked to asthma and COPD.
- Weight loss. In the case of obesity hypoventilation syndrome, significant weight loss may be necessary to reduce abnormal compression of the lungs.
- Provide mechanical ventilation through oxygen supplementation. Additional oxygen may be provided to alleviate the low oxygen level in the blood.
- Manage hyperkalemia through the use of Kayexalate. Acidosis causes potassium to move from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the gastrointestinal tract.
- Maintain adequate hydration. Provide intravenous fluids and electrolytes as ordered.
Respiratory Alkalosis
Respiratory alkalosis can result from hyperventilation since the lungs excrete too much carbonic acid which increases pH. Since respiratory alkalosis occurs quickly, the kidneys do not have time to compensate. Neurological symptoms such as confusion, paresthesias, and cell membrane excitability occur when the blood pH, CSF, and ICF increases acutely.
Risk Factors
Causes of hyperventilation include:
- Panic. Panic attacks and anxiety are the most common causes of hyperventilation.
- Hyperthermia. Fever may manifest as hyperventilation. The exact mechanism is not known but is thought to be due to carotid body or hypothalamic stimulation by the increased temperature.
- Brainstem damage. Central neurogenic hyperventilation (CNH) is the human body's response to reduced carbon dioxide levels in the blood. This reduction in carbon dioxide is caused by the contraction of cranial arteries from damage caused by lesions in the brain stem.
- Metabolic acidosis. Hyperventilation occurs most often as a response to hypoxia, metabolic acidosis, increased metabolic demands, pain, or anxiety.
- Diabetic ketoacidosis (DKA). The only known compensatory response to metabolic acidosis in DKA is hyperventilation with consecutive respiratory alkalosis.
- Pregnancy. Progesterone levels are increased during pregnancy. Progesterone causes stimulation of the respiratory center, which can lead to respiratory alkalosis.
- Salicylate toxicity. Salicylate toxicity causes respiratory alkalosis and, by an independent mechanism, metabolic acidosis.
Signs and Symptoms
Hyperventilation is a sign that respiratory alkalosis is most likely to occur. However, low carbon dioxide levels in the blood also have a number of physical effects, including:
- Numbness. Increased neuromuscular irritability in which a person loses feeling in a particular part of their body.
- Tingling sensation. Prickling sensation that is usually felt in the hands, arms, legs, or feet, but can also occur in other parts of the body.
- Palpitations. Palpitations are the perceived abnormality of the heartbeat characterized by awareness of cardiac muscle contractions in the chest.
- Tetany. Tetany or tetanic seizure is a medical sign consisting of the involuntary contraction of muscles.
- Convulsions. A medical condition where body muscles contract and relax rapidly and repeatedly, resulting in uncontrolled actions of the body.
- Signs and symptoms of hypokalemia and hypocalcemia. Persistent respiratory alkalosis can induce secondary hypocalcemia and hypokalemia that may cause cardiac arrhythmias, conduction abnormalities, and various somatic symptoms such as paresthesia, hyperreflexia, convulsive disorders, muscle spasm, muscle twitching, positive Chvostek's sign, and tetany.
Management of Respiratory Alkalosis
The treatment for respiratory alkalosis depends on the underlying cause. Treating the condition is a matter of rising carbon dioxide levels in the blood. The following strategies and tips are useful for respiratory alkalosis caused by over-breathing due to panic and anxiety.
- Breathe into a paper bag. Breathing through a paper bag fills it with carbon dioxide helping in inhaling exhaled air back into the lungs.
- Treat underlying condition:
- Medications. Administering an opioid pain reliever or anti-anxiety medication to reduce hyperventilation.
- Relaxation techniques. Breathing exercises that help relax and breathe from the diaphragm and abdomen, rather than chest wall.
- Safety. Stay with the patient.
- Lavage. After massive aspirin ingestions, aggressive gut decontamination is advisable, including gastric lavage.
- Correction of hypokalemia and hypocalcemia.
- Oxygenation as indicated. Providing oxygen to help keep a person from hyperventilating.
Metabolic Acidosis
Metabolic acidosis is when there is a decrease in bicarbonates and a buildup of lactic acid occurs. This happens in diarrhea, ketosis, and kidney disorders. It has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids.
Risk Factors
- Diabetic Ketoacidosis (DKA). DKA develops when substances called ketone bodies (which are acidic) build up during uncontrolled diabetes. DKA occurs mostly in Type 1 Diabetes Mellitus (DM).
- Chronic Renal Failure (CRF). This is due to reduced tubular bicarbonate reabsorption and insufficient renal bicarbonate production in relation to the number of acids synthesized by the body and ingested with food.
- Chronic Hypoxia. With chronic hypoxia, metabolic and hypercapnic acidosis develop along with considerable lactate formation and pH falling to below 6.8.
- Obesity. Obesity, especially in conjunction with insulin resistance, can increase metabolic acidosis and thus result in a reduction of urinary citrate excretion.
- Diarrhea. Loss of bicarbonate stores through diarrhea or renal tubular wasting leads to a metabolic acidosis state characterized by increased plasma chloride concentration and decreased plasma bicarbonate concentration.
- Dehydration. Electrolyte disturbances caused by prolonged vomiting or severe dehydration can cause metabolic acidosis.
- Aspirin Toxicity. Aspirin overdose causes the body to not produce ATP, leading to anaerobic metabolism with consequent raised lactate and ketone bodies. Acute aspirin or salicylates overdose or poisoning can cause initial respiratory alkalosis through metabolic acidosis ensues thereafter.
- Methanol Poisoning. Significant methanol ingestion leads to metabolic acidosis, which is manifested by a low serum bicarbonate level. The anion gap is increased secondary to high lactate and ketone levels. This is probably due to formic acid accumulation.
Signs and Symptoms
- Altered level of consciousness
- Confusion
- Disorientation
- Lack of appetite
- Coma
- Jaundice
Management of Metabolic Acidosis
Patients with arterial blood gas indicating metabolic acidosis are managed and treated by:
- Sodium bicarbonate. Indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest, and severe primary lactic acidosis.
- Treat the underlying condition.
- Hydration for diabetic ketoacidosis. The major treatment of this condition is the initial rehydration.
- Dialysis for chronic renal failure. The control of metabolic acidosis in hemodialysis is mainly focused on the supply of bicarbonate during the dialysis sessions.
- Use of diuretics.
- Initiate safety measures.
- Kayexalate. Acidosis causes potassium to move from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the gastrointestinal tract.
Metabolic Alkalosis
Metabolic alkalosis occurs when bicarbonate ion concentration increases, causing an elevation in blood pH. This can occur in excessive vomiting, dehydration, or endocrine disorders.
Risk Factors
- Vomiting. Vomiting causes metabolic alkalosis by the loss of gastric secretions, which are rich in hydrochloric acid (HCl). Whenever a hydrogen ion is excreted, a bicarbonate ion is gained in the extracellular space.
- Sodium bicarbonate overdose. Administration of sodium bicarbonate in amounts that exceed the capacity of the kidneys to excrete this excess bicarbonate may cause metabolic alkalosis.
- Hypokalemia. Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH.
- Nasogastric suction. Just like in vomiting, nasogastric (NG) suction also generates metabolic alkalosis by the loss of gastric secretions, which are rich in hydrochloric acid (HCl).
Signs and Symptoms
Metabolic alkalosis may not show any symptoms. People with this type of alkalosis more often complain of the underlying conditions that are causing it. These can include:
- Numbness
- Vomiting
- Diarrhea
- Swelling in the lower legs (peripheral edema)
- Fatigue
- Tingling sensation
- Agitation
- Disorientation
- Seizures
- Coma
Management of Metabolic Alkalosis
- Antiemetic. In the case of vomiting, administer antiemetics, if possible.
- Ammonium chloride. Ammonium chloride is a systemic and urinary acidifying agent that is converted to ammonia and hydrochloric acid through oxidation by the liver. Intravenous (IV) ammonium chloride is a treatment option for severe cases of metabolic alkalosis.
- Acetazolamide (Diamox). Acetazolamide also appears to be safe and effective in patients with metabolic alkalosis following treatment of respiratory acidosis from exacerbations of chronic obstructive pulmonary disease (COPD).
Arterial Blood Gas Interpretation Quiz
If you need to practice your new skills acquired here, check out our Arterial Blood Gas Interpretation for NCLEX (40 Questions)
References and Sources
The following sources are used as references for this guide. You may find them interesting for your additional reading:
- Barnette, L., & Kautz, D. D. (2013). Creative ways to teach arterial blood gas interpretation.Dimensions of Critical Care Nursing,32(2), 84-87.
- Samuel, R. (2018). A Graphical Tool for Arterial Blood Gas Interpretation using Standard Bicarbonate and Base Excess.Indian J Med Biochem,22(1), 85-89.
- Sood, P., Paul, G., & Puri, S. (2010). Interpretation of arterial blood gas.Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine,14(2), 57.
- Williams, A. J. (1998). Assessing and interpreting arterial blood gases and acid-base balance.Bmj,317(7167), 1213-1216.
- Verma, A. K., & Roach, P. (2010). The interpretation of arterial blood gases.Aust Prescr,33(4), 124-129.
fitzgeraldwispond.blogspot.com
Source: https://nurseslabs.com/arterial-blood-gas-abgs-interpretation-guide/
0 Response to "Acid Base Balance Made Easy for Nurses"
Post a Comment